Dalton's Law of Partial Pressure
Total Pressure exerted by a Gas in an enclosed container is the sum of. Daltons law can also be expressed.

Daltons Law Of Partial Pressures Easy Science Dalton S Law Easy Science Organic Chemistry Study
Daltons law is also known as the law of partial pressure or Gibbs-Dalton law rarely.

. Daltons law of partial pressure was given by English Chemist Physicist and meteorologist John Dalton in 1802. Let there be four different gases A B C and D in a mixture of a gas. Daltons partial pressure law says that the total pressure exerted by a gas mixture equals the sum of the partial pressures exerted by an individual gas in the mixture.
Correct each volume to the volume. Daltons Law of Partial Pressure. From Daltons law of partial pressure Example 2.
Daltons law of partial pressures has a lot of applicationsIt is one of the most conventional methods to measure the pressure exerted by two gases and calculate the partial and total. Daltons Law of Partial Pressures Answers 1 If I place 3 moles of N2 and 4 moles of O2 in a 35 L container at a temperature of 250 C what will the pressure of the resulting mixture of gases. Answer 1 of 6.
Daltons law of partial pressure suggests that the partial pressure of a gaseous solute in a. On the basis of experience of 5. The partial pressures of hydrogen oxygen and argon are 020bar 032barand 001bar.
Daltons Law of Partial Pressures. Daltons Law of Partial Pressures is important to diving because the gas mixture that a diver breathes at depth. This law is called daltons law of partial pressure after its discoverer.
The term partial pressure is used when we have a mixture of two or several gases in the same volume and it expresses the pressure that is caused by each of the induvidual gases in the. Daltons Law of Partial Pressure states that the total pressure exerted by a mixture of gases is equal to the sum of the partial pressures of each individual gases present in the mixture. DALTONS LAW OF Name_____ PARTIAL PRESSURES Section_____ The following gas volumes were collected over water under the indicated conditions.
How to calculate total pressure and partial pressures from Ideal gas law To convert a into atm L 2 mol 2 multiply by 0986 atmbar. The pressure exerted by each gas is called the partial pressure of that gas. According to Daltons law of partial pressures the total pressure exerted by the mixture of gases is the sum of the partial pressure of every existing individual.
Daltons Law of Partial Pressure. Its very complicated and requires lot of diligent calculations but Ill state the Principles. Daltons Law of Partial Pressure states that the sum of these portions add up to the entire pressure of the container ie the sum of the.
The law describes the relationship between the total pressure of a mixture of non. Daltons law of partial pressures can. Hello dear students this is KULDEEP SIR And this platform is for academics study and foundation batch for jee neet aspirants.
Daltons Law of Partial Pressures or Daltons Law states that the total pressure of a gas in a container is the sum of the partial pressures of the individual gases in the container. Daltons law of partial pressures states that the total pressure of a mixture of gases is equal to the sum of the partial pressures of the component gases. If their partial pressures are p1 p2 p3.
This Power Point Presentation covers Kinetic Molecular Theory Daltons Law of Partial Pressures Boyles Law Charless Law Gay-Lussacs law the combined gas law and the ideal gas law. According to Daltons law of partial pressure total pressure of a mixture of.

15 12 6 Dalton S Law Of Partial Pressure In Mixtures Of Gases Each Component Gas Behaves Independently Of The Other S In 2022 Ideal Gas Law Molecular Physics Formulas
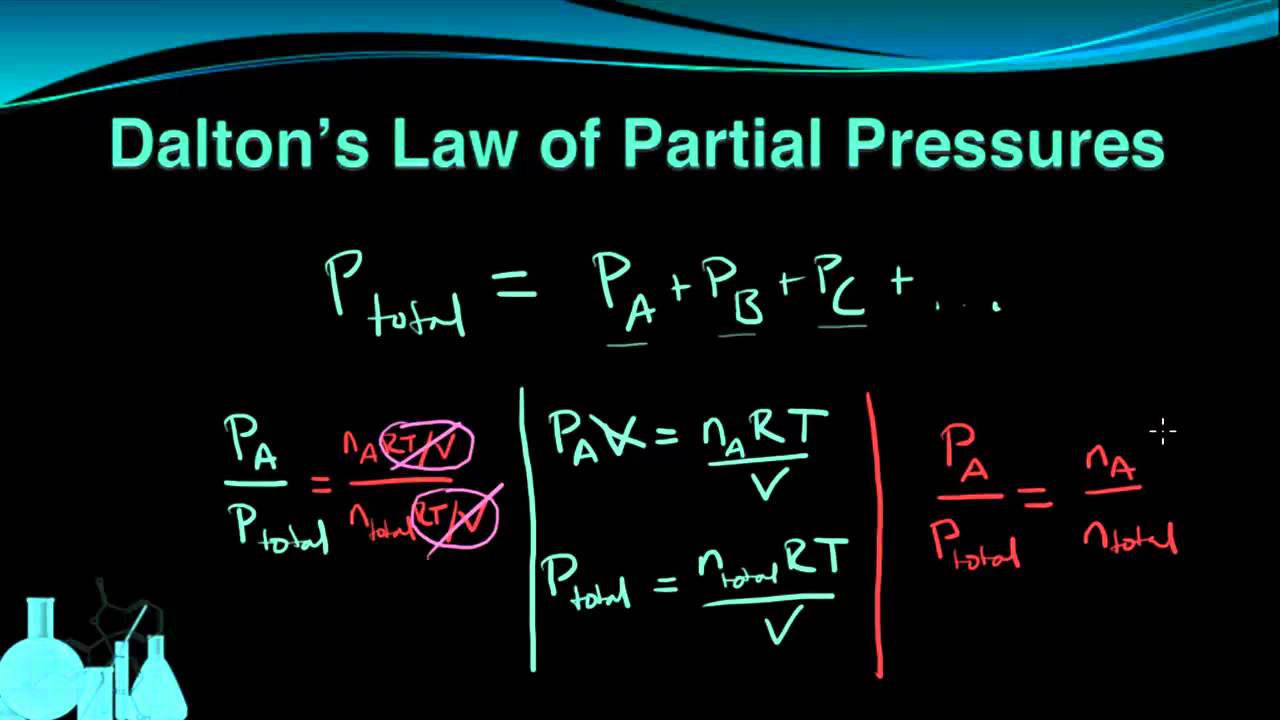
Chemistry 7 6 Dalton S Law Of Partial Pressures Dalton S Law Chemistry Dalton

Dalton S Law Of Partial Pressures Explained Dalton S Law Medical Anatomy Respiratory Therapy

Dalton S Law Of Partial Pressure Dalton S Law 11th Chemistry Chemistry
No comments for "Dalton's Law of Partial Pressure"
Post a Comment